Студопедия КАТЕГОРИИ: АвтоАвтоматизацияАрхитектураАстрономияАудитБиологияБухгалтерияВоенное делоГенетикаГеографияГеологияГосударствоДомЖурналистика и СМИИзобретательствоИностранные языкиИнформатикаИскусствоИсторияКомпьютерыКулинарияКультураЛексикологияЛитератураЛогикаМаркетингМатематикаМашиностроениеМедицинаМенеджментМеталлы и СваркаМеханикаМузыкаНаселениеОбразованиеОхрана безопасности жизниОхрана ТрудаПедагогикаПолитикаПравоПриборостроениеПрограммированиеПроизводствоПромышленностьПсихологияРадиоРегилияСвязьСоциологияСпортСтандартизацияСтроительствоТехнологииТорговляТуризмФизикаФизиологияФилософияФинансыХимияХозяйствоЦеннообразованиеЧерчениеЭкологияЭконометрикаЭкономикаЭлектроникаЮриспунденкция |
CH-ACIDIC PROPERTIES OF HETEROFUNCTIONAL COMPOUNDSAliphatic compounds of the general formula X-CH2-Y, in which the substituents X and Y represent electron-withdrawing groups, reveal the property of CH-acids. This is a result of polarization of the C-X and C-Y bonds with subsequent polarization of the C-H bond. Amino acids, hydroxy acids and oxo acids with the second functional group at the atom C-3 (or the β position) belong to compounds of this type. Elimination Reactions Elimination reactions take place readily on heating β-hydroxy or β-amino acids. Both types of the acids form α,β-unsaturated acid, releasing water or ammonia, respectively:
β-hydroxy acid Citric acid which is simultaneously both the α and β hydroxy acid undergoes similar elimination. This reaction is a chemical analogue of one of the steps in the Krebs cycle.
Thermal dehydration of malic acid analogously yields maleic acid.
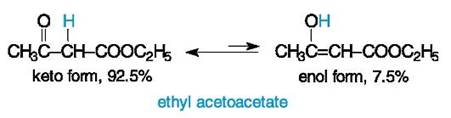
Keto-Enol Tautomerism Keto-enol tautomerism is more pronounced for compounds that have a strong CH-acidic site. This is observed in β-oxo carboxylic acids and their derivatives. The most known representative in this series is ethyl acetoacetate, CH3C(O)CH2COOC2H5, commonly named acetoacetic ester. It has the pKa of 10.7 (for a proton of the CH2 group) which is comparable with phenolic acidity, and it is at least 106 times more acidic than alcohols.
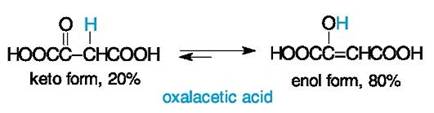
A structure of acetoacetic ester was an object of discussions of many years' standing. This compound first obtained in 1863 was assigned the structure of a hydroxy unsaturated ester (i. e. the enolic structure in modern terminology) because the product reacted with sodium like alcohols and with bromine like alkenes. Shortly after, it was shown that acetoacetic ester also reacted as a typical ketone. Both forms of acetoacetic ester were isolated under specific conditions some decades later. A long-standing controversy finished at the beginning of the 20th century when theconcept of tautomerism was adopted in organic chemistry. It is interesting that A. Butlerov first predicted, on the basis of his structure theory, the possibility of a dual reactivity of some compounds and of tautomerism as a reversible isomerization. But this phenomenon was first demonstrated by A. Baeyer (in the 1880's) for another type of a reversible transformation, so-called lactim-lactam tautomerism that will be discussed in the next chapter. Oxalacetic acid is one of the most important carboxylic acids in many biochemical processes. Despite its common name that corresponds to an oxo acid, this compound is a rather unsaturated acid because of the predominance of the enol form in the tautomeric equilibrium.
Phosphoenolpyruvic acid is an example of an enolic compound in living systems. It is produced in the glycolysis process and represents a phosphate of pyruvic acid in the enol form. Phosphoenolpyruvic acid is an energy-rich compound that eliminates energy on its transformation into pyruvic acid. The energy thus released is then accumulated in adenosine triphosphate (ATP) produced from adenosine diphosphate (ADP):
It should be mentioned that pyruvic acid itself contains only negligible quantities of the enol form. Problem 5.Which of the following compounds can exist in the enol form: (a) 2-oxopentanoic acid; (b) 2-oxopentanedioc acid; (c) 3-oxopentanoic acid; (d) 3-oxopentanedioc acid; (e) 2,5-hexanedione? Write the enol structure(s). |
||
|
Последнее изменение этой страницы: 2018-05-10; просмотров: 185. stydopedya.ru не претендует на авторское право материалов, которые вылажены, но предоставляет бесплатный доступ к ним. В случае нарушения авторского права или персональных данных напишите сюда... |