Студопедия КАТЕГОРИИ: АвтоАвтоматизацияАрхитектураАстрономияАудитБиологияБухгалтерияВоенное делоГенетикаГеографияГеологияГосударствоДомЖурналистика и СМИИзобретательствоИностранные языкиИнформатикаИскусствоИсторияКомпьютерыКулинарияКультураЛексикологияЛитератураЛогикаМаркетингМатематикаМашиностроениеМедицинаМенеджментМеталлы и СваркаМеханикаМузыкаНаселениеОбразованиеОхрана безопасности жизниОхрана ТрудаПедагогикаПолитикаПравоПриборостроениеПрограммированиеПроизводствоПромышленностьПсихологияРадиоРегилияСвязьСоциологияСпортСтандартизацияСтроительствоТехнологииТорговляТуризмФизикаФизиологияФилософияФинансыХимияХозяйствоЦеннообразованиеЧерчениеЭкологияЭконометрикаЭкономикаЭлектроникаЮриспунденкция |
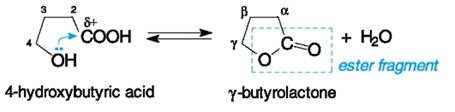
INTERACTION OF DIFFERENT GROUPS IN HETEROFUNCTIONALCOMPOUNDS Many functional groups listed in Table 1 can affect each other, especially if one of these groups is a carboxyl or carbonyl group. Two types of interaction are possible: intermolecular or intramolecular; the latter occurs when two functional groups occupy favourable positions for such a reaction. Intramolecular Reactions Lactones and lactams.Let us begin consideration from a familiar reaction such as ester formation. Fundamentally, the nucleophilic OH group of hydroxy acids can react with its own carboxyl group. Cyclic esters having the generic name lactones would be expected in this case. Indeed, cyclization occurs if a thermodynamically stable fiveor six-membered cycle is formed. A molecule takes a claw-shaped conformation to make a better contact of both functional groups. This becomes possible for γ-hydroxy and δ-hydroxy carboxylic acids which undergo internal esterification (lactonization) in an acidic medium or on slight heating or even spontaneously, though slowly, in aqueous solution. For example:
Principally similar transformation proceeds with γ-amino and δ-amino acids. Cyclic amides called lactams are the cyclization products.
The main difference in these two cyclization reactions is that lactams are never obtained spontaneously or under acidic conditions because amino acids form ammonium salts in the presence of acids. Lactam formation is a thermal reaction. Problem 2.Write equations for the formation of: (a) δ-valerolactone; (b) γ-valerolactam from the corresponding hydroxy and amino acid. Nomenclature of lactones and lactams. Some common names, derived from the trivial names of non-hydroxylated carboxylic acids, are permitted by the IUPAC rules. Such names were used in the text and in Problem 11.2. Systematically, lactones formed from aliphatic acids are named by adding the suffix -olide to the name of the parent hydrocarbon. A locant is added to define the position of ring closure. Thus, γ-butyrolactone should be named as «4-butanolide», and δ-valerolactone - as «5-pentanolide». The same principles, but with the use of the suffix -lactam, are applied to nomenclature of lactams. For example, γ-valerolactam and δ-valerolactam have systematic names «4-pentanelactam» and «5-pentanelactam», respectively. Cyclic hemiacetals.If an aldehyde or a ketone contains a hydroxyl group at an appropriate distance (at the C-4 or C-5 atom for aldehydes), the carbonyl and hydroxyl groups may react with each other. The molecule takes a conformation in which both functional groups are favourably located. The result of intramolecular nucleophilic addition is the formation of a cyclic hemiacetal. For example, 4-hydroxybutanal exists as an equilibrium mixture of two forms with predominance (about 94%) of the cyclic one.
In general, fiveand six-membered cyclic hemiacetals are more stable than their open-chain counterparts. As we will see later, a cyclic hemiacetal form is the element of a carbohydrate structure.  Problem 3.Write an equation for the formation of cyclic hemiacetal from 5-hydroxy-2-methylhexanal. Which form is predominant in the equilibrium mixture? Intermolecular Reactions Cyclization reactions.The formation of a threeor four-membered cycle from the α- or β-substituted acid is unfavourable because of a great angle strain in small cycles. Nevertheless, α-hydroxy acids and α-amino acids can react intermolecularly. Lactic acid, for example, undergoes intermolecular esterification on heating with the formation of a dimeric product which, in its turn, converts into more stable six-membered cyclic diester (ester units are coloured in the equation below). The generic name of such cyclic diesters is lactides.
Problem 4.Explain why α-hydroxy and γ-hydroxy acids produce quite different products on heating. Illustrate this by the examples of 2-hydroxyand 4-hydroxypentanoic acids. Four-membered cyclic esters and amides, β-lactones and β-lactams, cannot be prepared from the corresponding acids on heating for the reason of their low stability. Nevertheless, they are known and have been found in nature, for example, in antibiotics of penicillin and cephalosporine series. The general structures of the socalled β-lactam antibiotics are given below (β-lactam fragments are outlined):
All the cyclic esters and amides mentioned above (i. e. lactones, lactides, and lactams) can be hydrolyzed just as «ordinary» carboxylic acid derivatives with the ring opening and the formation of the starting heterofunctional compounds; for example:
Complexing properties.These are another characteristic of heterofunctional compounds. Their complexing (or chelating) ability is based on a tendency to form a stable fiveor six-membered cycle in the reaction with some metal ions (especially with Cu2+ and Ni2+). For example, insoluble copper(II) hydroxide reacts with 1,2-diols with the formation of dark blue coloured solution.
Similar complex salts are produced when treating α-amino alcohols and α-amino acids with Cu(OH)2. These reactions are used as a colour test for the detection of the vicinal diol fragment in the molecule. |
||
|
Последнее изменение этой страницы: 2018-05-10; просмотров: 181. stydopedya.ru не претендует на авторское право материалов, которые вылажены, но предоставляет бесплатный доступ к ним. В случае нарушения авторского права или персональных данных напишите сюда... |